What is FDA Warning Letters ?

USFDA warning letters explained in this blog. Every year, the FDA issues hundreds of warning letters along with inspections of manufacturing facilities. You should take the warning letters seriously because they act as a precursor to a worse failure. You should always take proper corrective and preventative actions accordingly and make a comprehensive gap analysis based on the mentioned requirements to be fully compliant.
FDA warning letters typically follow inspections where deviations or violations of regulations, such as those related to cGMP, data integrity etc. During inspections, auditors document these deviations using Form 483. While Forms 483 and FDA Warning Letters are distinct, the warning letter often follows as the next step in the regulatory process..
The FDA warning letter describes the violation and the potential problem, followed by specifying what evidence the manufacturer must present to document the addressed concern.
When a manufacturer significantly violates FDA regulations, they receive a Warning Letter from the FDA outlining the violation, such as poor manufacturing practices or inaccurate product claims. The letter mandates correction within a specified timeframe. FDA monitors the company’s corrective actions for adequacy. Subsequent interactions between the FDA and the recipient may impact the issues raised in the letter. Warning Letters are issued for serious violations or insufficient responses to FDA 483s, potentially leading to regulatory action if not resolved effectively.
Example: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters
What is FDA Form 483?

The FDA Form 483, officially known as “Notice of Inspectional Observations” and commonly referred to as “483,” is issued at the end of an on-site inspection of regulated facilities by the FDA field investigator in response to deficiencies in the quality system or conditions that violate the Food, Drug, and Cosmetic Act.
The 483 does not represent an FDA determination of violations; it is intended to guide corrective actions to be taken by the manufacturer based on inspection. When a manufacturer receives a 483, they must respond FDA by understanding its significance in pharmaceuticals to correct the issue to avoid a warning letter. The FDA 483 form serves as a tool to assist manufacturers in understanding and correcting compliance issues and is the starting point of communication between the FDA and the facility.
Understanding Trends of USFDA Warning Letters
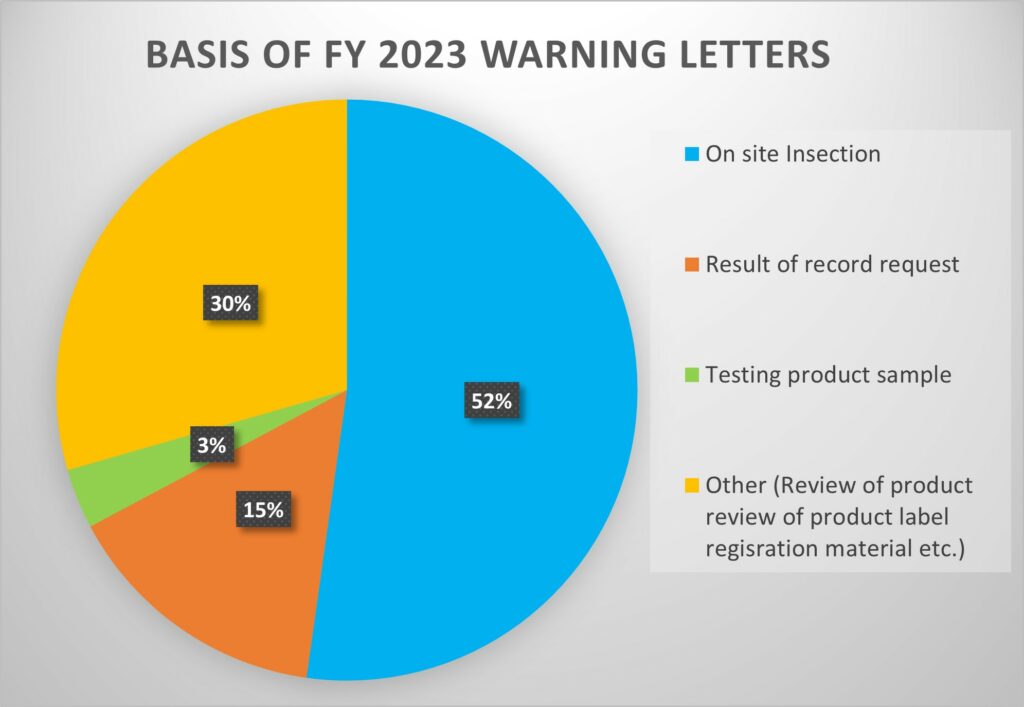
Based on data provided on FDA website, https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/compliance-actions-and-activities/warning-letters
In fiscal year 2023 (FY23), the U.S. FDA issued 180 warning letters to drug and biologics manufacturers. Among these, it happened as below,
- 94 were based on on-site inspections,
- 27 were prompted by records requests,
- 6 were originated from product sample testing
- Others related to product labels, registration materials, and/or websites.
This marked an increase compared to the 165 warning letters issued in FY22, with 74 from inspections.
The most common citations in FDA warning letters for the fiscal year 2023
An analysis of citations within the inspection-based warning letters revealed FDA’s top citations for FY2023. Now, as manufacturer we can learn from others mistake and plan internal audit to check readyness of factory and also solutions for FDA warning letter citations and discuss how to address FDA warning letters
- 21 CFR 211.100(a): This section is highlighted due to failure to establish adequate written procedures for production and process control.
- 21 CFR 211.22: This section is highlighted due to failure of quality control to exercise its responsibility to ensure drug products manufactured are in compliance with cGMP.
- 21 CFR 211.84: This section is highlighted due to failure to test and approve or reject components, drug product containers, and closures ). This citation talks about Testing and Approval Procedures, sampling criteria, procedures, including container cleaning and contamination prevention batches of components, drug containers, and closures. Sample Identification and Handling and their examination and testing requirements.
- 21 CFR 211.192: This section is highlighted due to failure to thoroughly investigate any unexplained discrepancy or failure of a batch or any of its components to meet any of its specifications whether or not the batch has already been distributed) . This citations talk that drug production and control records, including packaging and labelling, must be reviewed and approved by the quality control unit before batch release or distribution.
- 21 C.F.R. 211.165: This section is highlighted due to failure to test batch before release for distribution. For each batch of drug product, there shall be appropriate laboratory determination of satisfactory conformance to final specifications for the drug product. Each drug product batch must undergo laboratory testing to ensure conformity with final specifications.
Analyzing top data integrity findings in FDA warning letters
Data integrity findings are taken very seriously by the FDA. Deficiencies can erode the trust between the FDA and a company, resulting in FDA 483s, warning letters, import alerts, injunctions and in extreme cases, the FDA invoking the application integrity policy. Not surprisingly, the FDA is getting more aggressive with enforcement. Topping the list was the theme of incomplete or missing records.
- A lack of access control to IT systems including appropriate administrative rights and the use of shared accounts.
- Deleting or destroying original Good Manufacturing Practice (GMP) records was a theme. Citations included analysts deleting data on electronic records as well as finding sample notebooks and test records in the trash.
- Inappropriate manual integration where samples are reprocessed multiple times with no justification and only one set of data was reported.
- Audit trail issues were observed as systems were without audit trail capability and audit trails being disabled by users, to audit trails not being reviewed to detect deletion or manipulation of data.
- Login, common password sharing
- QMS excel spreadsheets not validated, or password protected
- Backup, restore not available, lack of traceability to individuals recording quality data on paper-based records
- Original quality records missing, Incomplete quality records
Need help with Consulting for FDA compliance

How to respond to 483 observation and Warning Letters ?
Steps to respond to FDA letters is really critical and unlike an FDA Form 483, a Warning Letter carries higher stakes. Failing to provide a satisfactory response to an FDA 483 may result in a Warning Letter, but an inadequate response to a Warning Letter can lead to severe enforcement actions.
These actions can significantly impact your ability to market products, including preventing international market access, seizures, injunctions, and potential criminal prosecution. For foreign manufacturers, receiving a Warning Letter can result in placement on the import hold list, effectively barring products from the U.S. market.
FDA warning letter response plan to a Warning Letter should offer actual and to the point response to obeservation raised in Form 483 and warning letter along with steps for improving compliance with FDA regulations. Please finds below essentials steps for How to address FDA warning letters ?
- Through certified mail, inform the FDA you intend to respond to the Warning Letter within 15 business days. This should be a simple correspondence meant only to show you intend to respond by the deadline.
- Once you’ve informed the FDA you intend to resolve the issues, it’s time to decide whether you’ll handle recovery on your own, or seek out an experienced third party for assistance.
- If you’re confident you have the right personnel on staff, gather key individual to lead the team. This person must have a comprehensive understanding of the problems cited and a clear idea of how to fix them.
- If you have any doubt in your ability to completely solve the problems internally, find an experienced third-party firm that has a track record of success.
- Define a list of the actions that either will be or were accomplished for each FDA observation general heading and specific example.
- Below are typical examples of actions that will be responsive to FDA observations. Determine which of these items are applicable and appropriate. List actions for each FDA observation and example. Examples: Evaluate product impact, remove product, place product on hold, provide procedure, conduct training, perform audits, consider expert advice, open CAPA, provide documents, validate methods, validate processes, complete system validation.
- Provide your response to FDA in a manner that is easy to understand and navigate. Response should contain Cover letter – Body of the response – List of attachments – Table of accomplishments etc.
- Cover letter should contain who will be the primary point of contact and signing your response letter. Cover letter should contain reason for the letter and defining terms, Management’s commitment and/or warning letter issues, addressing management responsibilities, requesting a meeting with FDA for systemic deficiencies or health risk issues, Identifying points of disagreement, Introducing appendices and commitment for next update response.
- Body of the response should contain systemic corrections for underlying issues, corrective actions for specific instances, steps for identifying, examining, and correcting similar problems, Impact assessment on manufactured products interim compliance measures, Realistic timelines for corrections, attachments verifying implemented actions.
- Provide what FDA wants to see in Appendix 2 -list of attachments
- Provide Table of Actions/Accomplishments in For Appendix 3 , use a table containing the number of each FDA 483 observation (or warning letter observation) and a brief description of the completed and planned actions with timeline. Find nice Template available for FDA 483/ Warning letter from sourse green light Guru Website
- Once they are satisfied your plan is adequate, you will be informed of the date for a follow-up inspection and closeout meeting. Although you may request to meet with the FDA to discuss your recovery plan, don’t rely on regulators to offer advice or meaningful insight into what you should do.
Lessons to learn
Anyone who receives an FDA Warning Letter must know that there is extreme distress. Now it’s time to use all our energy to get to the root of the problem.
Warning letters and inspection data are crucial for identifying regulatory focus areas and weaknesses in the quality system. With a sustained focus on GMP violations and an overall rise in citations in the pharmaceutical industry, heeding FDA warning letters is essential. Proactively avoiding them not only ensures compliance but also saves costs and prevents potential failures.
Frequently Asked Questions (FAQ'S)
A: An FDA Form 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed any conditions that in their judgment may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts. FDA investigators are trained to ensure that each observation noted on the FDA Form 483 is clear, specific and significant. Observations are made when in the investigator’s judgment, conditions or practices observed would indicate that any food, drug, device or cosmetic has been adulterated or is being prepared, packed, or held under conditions whereby it may become adulterated or rendered injurious to health.
A: The FDA Form 483 notifies the company’s management of objectionable conditions. At the conclusion of an inspection, the FDA Form 483 is presented and discussed with the company’s senior management. Companies are encouraged to respond to the FDA Form 483 in writing with their corrective action plan and then implement that corrective action plan expeditiously.
A: No, it’s not. The FDA Form 483 is a report which does not include observations of questionable or unknown significance at the time of the inspection. There may be other objectionable conditions that exist at the firm that are not cited on the FDA Form 483. FDA investigators are instructed to note only what they saw during the course of the inspection. Companies are responsible to take corrective action to address the cited objectionable conditions and any related non-cited objectionable conditions that might exist.
A: FDA Form 483s are discussed with a company’s management at the conclusion of the inspection. Each observation is read and discussed so that there is a full understanding of what the observations are and what they mean.
A: The FDA Form 483 does not constitute a final Agency determination of whether any condition is in violation of the FD&C Act or any of its relevant regulations. The FDA Form 483 is considered, along with a written report called an Establishment Inspection Report, all evidence or documentation collected on-site, and any responses made by the company. The Agency considers all of this information and then determines what further action, if any, is appropriate to protect public health.
Useful links:
The FDA has provided the following guidance documents on FDA Form 483 and Warning Letters:
FDA Form 483 Frequently Asked Questions
Warning Letters
Inspection Observations
Understanding the Form FDA 483 Process and Timelines
https://www.fda.gov/media/162162/download
Trends : Source https://www.outsourcedpharma.com/doc/trends-in-fda-fy2023-inspection-based-warning-letters-0001
Unveiling GMP Inspections: Key Deficiencies and Guidelines