Introduction
Introduction: According to the FDA, “the term qualification refers to activities undertaken to demonstrate that utilities and equipment are suitable for their intended use and perform properly. These activities necessarily precede manufacturing products at the commercial scale.”
Qualification can be further broken down into three phases: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Validation, and the qualification steps involved, are covered by various parts of 21 CFR 211 (pharmaceuticals) and 21 CFR 820 (medical devices), and are enforced by the FDA
This equipment sits at different points of the supply chain, from complex, futuristic laboratories to Amazon-esque packing facilities. They have one thing in common, though — a failure of any one of these pieces of equipment at any point in the chain can result in a hazardous product failure — mislabeled products, improper dosage, improper formulation, unsealed or unsanitary products, and more.
The message is clear — pharmaceutical companies need to pay the same kind of attention to quality assurance in the installation and use of equipment as they do in the formulation and distribution of their life-saving products — drugs, vaccines, and therapeutic products.
The gold standard of equipment quality assurance is abbreviated IQ OQ PQ. This process applies quality assurance standards to critical equipment at three stages of the installation process.IQ OQ PQ is a key hurdle a pharma company must clear to achieve regulatory compliance.
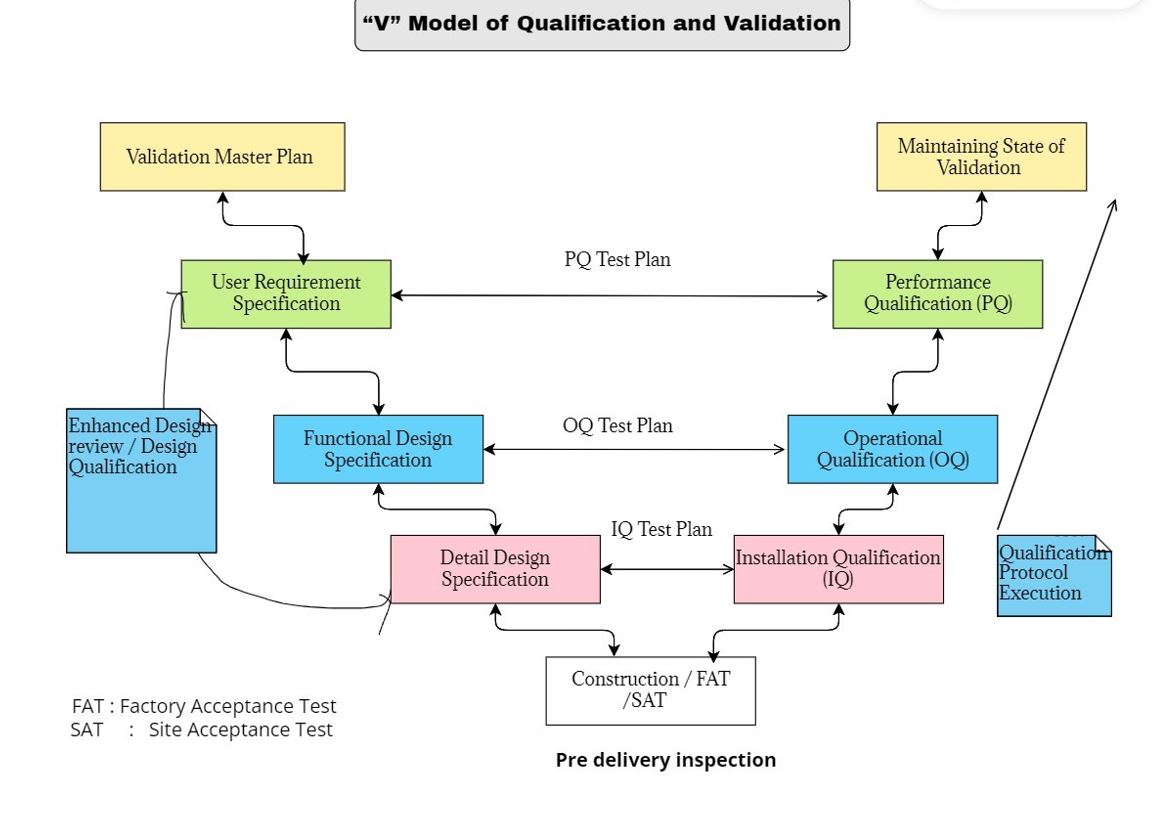
What is the V-Model ?
The V-model is a method used to visualize and compare the relationship between the user requirements, functional design and detailed design to the installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ) performed on them (see diagram below )
What is IQ OQ PQ in the Pharmaceutical Manufacturing Industry?
IQ OQ PQ stands for Installation Qualification, Operational Qualification, and Performance Qualification. These are 3 sequential processes used to confirm and document that the equipment, piping, instruments, and utilities (air, water, steam), etc are installed correctly, operate as intended, and perform consistently according to the design specifications.
The goal of these processes is to establish a documented evidence trail (paper or electronic) with multiple signatures from all relevant departments (Validation, Engineering, Maintenance, Calibration, Quality Assurance, etc,). This documentation proves to the (owners/clients or regulatory agencies) that the critical equipment ordered has been delivered, installed and configured correctly and that the system as a whole is working as per the engineering drawings and design specifications.
The system of GMP necessitates the practices of IQ, OQ and PQ for equipment qualification process. It helps manufacturers ascertain a consistent quality delivery from the equipment. Besides, all of these practices considerably cut down errors so that the product quality can be maintained in accordance with the relevant regulations and industry standards. You can conduct equipment qualification in-house or consult professionals who offer services for equipment validation.
Why do we need Qualification (IQ OQ PQ ) in Lifescience Industry?
Qualification is usually done by the engineering group, the validation team or any other person or group that is qualified and knowledgeable on the use and operation of the equipment, and has the training and experience to perform the tasks required.
Two main reasons.
1) The first reason is that it’s a legal requirement.
According to the Food and Drug Administration (FDA)
“Equipment used in the manufacture, processing, packing, or holding of a drug product shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its cleaning and maintenance.” – 21 CFR Title 211.63,
Within the EU, EudraLex – Volume 4 – Good Manufacturing Practice (GMP) provides more detailed guidance on qualification under Annex 15: Qualification and Validation
And while not regulation, ISPE Baseline Guide 5 Commissioning and Qualification (Second Edition) is a widely used guide in qualification that enjoys the support of numerous regulatory authorities.
2) The second reason is that when qualifying and validating a new plant or process
We are aware that even the slightest installation error or the most trivial problem with equipment performance can cascade and escalate into a serious product quality issue with deadly consequences for patients. This problem is especially acute with novel or new systems where there is zero track history of performance or failure and even tiny problems can lead to sick or dead patients.
What is Installation Qualification (IQ) ?
Installation Qualification (IQ) is a critical documented process essential for ensuring that equipment, piping, software, or instruments important to product quality are delivered, installed, and configured correctly. This process serves as a thorough verification against a pre-set checklist to ensure all installed components align with design specifications and installation standards, whether set by manufacturers or through an approved checklist.
Imagine installing a new machinery; IQ helps confirm that not only is the correct equipment delivered and made from specified materials, but it’s also installed in the right location with all connections, like pipework and electrical wiring, properly set up, instruments you specified in the detailed design specifications have been shipped and installed correctly. This detailed examination is typically conducted by specialized professionals such as Commissioning, Qualification, Validation (CQV) engineers, Commissioning and Qualification (C&Q) engineers, or Validation technicians.
This is the first step. IQ document of equipment shall be prepared by engineering department in coordination with user and QA. IQ is carried out to ensure that the premises supporting utilities and equipment have been built and installed in compliance with their approved design specification ( DQ) and manufacturers manual and recommendations.
What makes IQ successful?
Compares what was “specified” with what was “found” on site. Any noncompliance observed during installation qualification shall be recorded.
- Cross-checking delivery against packing lists and inspecting for damage.
- Ensuring equipment specifications match design and operational requirements.
- Confirming the accuracy of serial numbers, make, model, and that components are correctly identified.
- Verifying correct installation locations, connections to other units, and the installation of secondary and ancillary equipment.
- Checking energy or utilities supply and ensuring environmental and operating conditions meet manufacturer guidelines.
- Verifying software installations and recording firmware versions and serial numbers.
- Documenting calibration and validation dates and collecting all related manuals and certificates.

What is Operational Qualification (OQ) ?
Operational Qualification (OQ) ensures equipment performs consistently to operator needs and within manufacturer-defined ranges. OQ, essential for technical equipment and facility acceptance, mandates individual component testing as outlined in a test plan, with detailed performance documentation.
This phase, initiating post-IQ, confirms equipment operational reliability and adherence to operational specifications. Requalification, crucial after equipment modifications, major maintenance, or as part of quality assurance, focuses on assessing equipment attributes crucial to product quality, reinforcing OQ’s role in validating equipment’s contribution to final product integrity.
What makes Operation Qualification (OQ) successful?
Here we compares what was “specified” in the functional design with test results.
- After successful completion of IQ. OQ shall be performed to verify that the equipment, instrument, utility, and system, operates with respect to design specification, cGMP requirement.
- Tests and documents that the equipment and systems operate as intended and are within the operating ranges listed by the manufacturer.
- Operational testing is to be done whenever possible to challange the system to the limits of anticipated operating conditions, alarms interlock, basic operations as per functional specification of machinery.
- Typically, OQ shall be done without load. However, if the equipment can not be run without load, then load trials may be taken.
- OQ must also verify the performance of the equipment’s components, as applicable, such as motors, blowers, sensors, functioning interlocks, safety features etc.
- Any noncompliance observed during operational qualification shall be recorded.
- The completion of successful OQ should be allowed the finalization of standards operating and cleaning procedures, operator training and preventive maintenance requirement.
- In Protocol after testing plan add references, Deviation from predefined specification if any , change control if any.
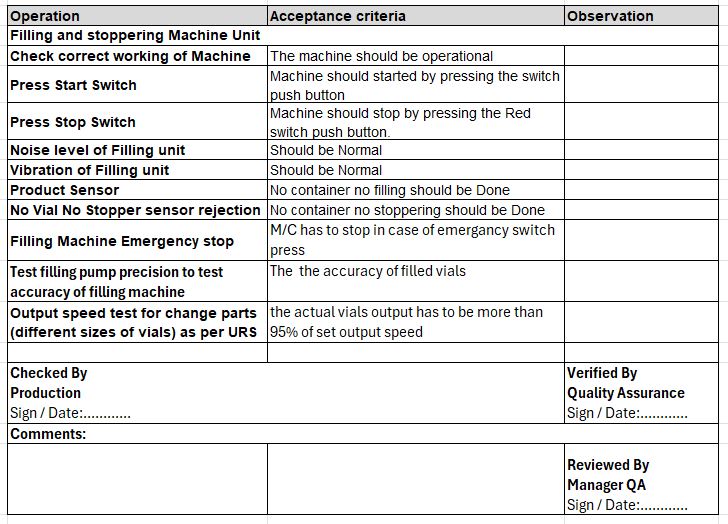
Example: Consider Vial filling Machine, lets check basic operation qualification test and acceptance criteria.
What is Performance Qualification (PQ) ?
Performance Qualification confirms that the equipment and systems meet the users’ needs and is fit for intended use as defined in the user requirements specification (URS).
It is the final step in equipment qualification.PQ should normally follow the successful completion of IQ and OQ. Through PQ, organizations can demonstrate with concrete evidence that their equipment not only meets design specifications and regulatory requirements but also consistently produces high-quality products under actual operating conditions.
For example, in the pharmaceutical industry, a PQ might involve running full production batches of a drug and closely monitoring the process to ensure that each pill meets stringent quality criteria, from composition to dissolution rate, under the full spectrum of production scenarios.
What makes Performance Qualification (PQ) successful?
Remember, below points while performing PQ,
- PQ verifies the URS (User requirement specification) with PQ test plan, Confirms and documents that the equipment and systems are fit for intended use as defined. It is the final step in equipment qualification.
- Tests should cover the operating range of the intended process with the equipment under load.
- It is much like Operational Qualification, as it tests the operational requirements of the equipment, but in this case, the equipment will contain a load or process filling medium to test real production scenario.
- In other words, you test the equipment while it’s being subject to “real-world” conditions – the conditions that the equipment will be subject to during batch production.
- This phase is hugely important as it combines the workings, forces and energy of the individual components of the equipment into one harmonious system. In doing so, this phase of qualification can identify faults such as
- Normally Pre-qualification requirements like SOP for operation, SOP for preventative maintenance, training records of validation teams.
- PQ demonstrate that the system perform as intended by repeatedly running the system on its intended schedules under normal operating conditions and worstcase conditions.
- For example: For Filling and stoppering machine. PQ help to access the machine capability to fill consistent volumes at various running speed, to check stoppering of vials through below Test plan with respect to URS.
Conclusion:
Ensuring equipment quality in pharmaceutical manufacturing involves IQ, OQ, PQ phases. These phases confirm correct installation, consistent performance, and meeting user needs. Regulatory requirements mandate this process, preventing hazards like mislabeling or improper dosages. Periodic requalification ensures ongoing compliance and quality. Expert guidance can streamline validation efforts and maintain robust, compliant systems. #PharmaceuticalManufacturing
Useful Link:
- EudraLex, Volume 4, EU Guidelines for Good Manufacturing Practice, Annex 15: Qualification and Validation
- 21 CFR Part 210 – Current Good Manufacturing Practice in Manufacturing. Processing, Packing, or Holding of Drug
- 21 CFR Part 211 – Current Good Manufacturing Practice for Finished Pharmaceuticals 4. TGA – PE009, the PIC/S guide to GMP for medicinal products 5. ICH Q9: Quality Risk Management, Step 5 version
- ISPE Baseline Guide Volume 5: Commissioning and Qualification
- ISPE GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems
- https://www.linkedin.com/in/marco-klinger-b86bb3151/recent-activity/all/
- https://zamann-pharma.com/2024/03/04/computer-validation-strategies/
- https://youtu.be/JrZ9cIrDX5s

Sagar Pawar
Computer System Validation Specialist



